There is a cycle to cycle variation in ovarian response and pre-hCG serum progesterone level: an analysis of 244 consecutive IVF cycles
- Sule Yildiz,
- Kayhan Yakin,
- Baris Ata &
- Ozgur Oktem
Scientific Reports volume 10, Article number: 15793 (2020) Cite this article
2088 Accesses
2 Altmetric
Metrics
Abstract
We aimed to answer one key question, that was not previously addressed as to whether serum progesterone (P4-hCG day) and its co-variates (estradiol (E2-hCG day) and the number of retrieved oocytes) of a given cycle can be predictive of the subsequent cycle when both cycles are consecutive and comparable for the stimulation protocol, gonadotropin dose and duration of stimulation. We analyzed such 244 consecutive (< 6 months) IVF cycles in 122 patients with GnRH agonist long protocol and found that P4, E2 and the number of retrieved oocytes significantly vary between the two cycles. Although P4 increased (ranging from 4.7 to 266.7%) in the 2nd cycle in 61 patients, E2 and the number of retrieved oocytes, which are normally positively correlated with P4 paradoxically decreased in the 41% and 37.7% respectively, of these same 61 patients. When a similar analysis was done in the 54 out of 122 patients (44.3%) in whom serum P4 was decreased in the 2nd cycle, the mean decrease in P4 was − 34.1 ± 23.3% ranging from − 5.26 to − 90.1%. E2 and the number of retrieved oocytes paradoxically increased in the 42.3% and 40.7% of these 54 patients respectively. P4 remained the same only in the 7 (5.7%) of these 122 patients. These findings indicate that late follicular phase serum P4 may change unpredictably in the subsequent IVF cycle. The changes are not always necessarily proportional with ovarian response of previous cycle suggesting that growth characteristics and steroidogenic activities of antral cohorts may exhibit considerable cycle to cycle variations.
Introduction
Serum progesterone (P4) level may elevate during late follicular phase of ovarian stimulation before ovulation is triggered and is considered a negative predictive factor for clinical outcome in assisted reproduction in both GnRH agonist and GnRH antagonist protocols1. It is not a true luteinization event because the elevations in serum P4 level occurs prior to hCG administration and is not associated with any premature LH surge. This phenomenon is generally observed in stimulated IVF cycles and shows significant correlation with the intensity of ovarian stimulation; hence patients with more growing follicles and retrieved oocytes have higher P levels. Interestingly, it was also documented that pre-ovulatory P4 elevations may also occur in up to 28% of natural cycles and reduce pregnancy rates. However, the mechanism of P4 rise in natural cycles is distinct from stimulated IVF cycles because there is no gonadotropin stimulation or multiple follicle development in the former. In agreement with the close link between the degree of ovarian stimulation with gonadotropins and serum pre-hCG P4 level in stimulated IVF cycles, we recently provided a molecular explanation for this phenomenon by showing that FSH stimulation itself promotes P4 synthesis and output from human granulosa cells without luteinization by up-regulating the expression and enzymatic activity of the enzyme 3β-hydroxy steroid dehydrogenase (3β-HSD), which converts pregnenolone to progesterone. Therefore, it is likely that pre-ovulatory P4 elevation is caused by the insufficiency of the ovary in handling the increased output of precursor steroids generated during multi-follicular development in FSH stimulated IVF cycles. To date, several clinical studies and meta-analyses showed a negative impact of P4 elevations before ovulation trigger on the chance of pregnancy in fresh embryo transfer IVF cycles
In most of the cases in stimulated IVF cycles, the risk for an elevation in serum P4 level at late follicular phase before ovulation trigger is closely related to the magnitude of ovarian stimulation, that is also reflected by E2 level on the hCG day, and the number of growing follicles > 14 mm on day 10 of stimulation and total and mature oocytes retrieved. Therefore, there are significant positive associations between P4 and these co-variates. However, one key question remains to be answered as to whether we should expect to see similar pre-hCG serum P4 levels and its co-variates in two consecutive IVF cycles comparable for gonadotropins, GnRH analog used, and duration of stimulation. We aimed to answer this question in this non-interventional, retrospective cohort data from a single center.
Material and methods
Study design
This study is a retrospective cohort analysis from a single center. It was approved by the Institutional Review Board (IRB) of Koc University (2015.206.IRB2.076). It is not a research study that involve human participation. Therefore, the need of the written informed consent was waived by the Institutional Review Board (IRB) of Koc University. All methods were performed in accordance with the relevant guidelines and regulations of the Institution. We reviewed the electronic database of 8724 IVF cycles that had been performed from 2008 to 2015 in Assisted Reproduction Unit, American Hospital of Istanbul, Turkey. A total of 122 women who had 244 consecutive IVF cycles within a 6-month interval following an unsuccessful cycle, using exactly the same ovarian stimulation protocol in both cycles were identified and included in this study. Patients who underwent stimulation more than 6 months apart or had ovarian surgery, systemic disease that could affect ovarian response to stimulation were excluded.
Ovarian stimulation and ovulation trigger
Pituitary down-regulation was induced with GnRH agonist leuprolide acetate started 7 days prior to the anticipated day of menstrual bleeding and continued until the day of hCG trigger. Recombinant FSH was started on cycle day three at a dose of 225–300 IU depending upon age, antral follicle count, anticipated or documented previous ovarian response, and body mass index. Ovulation was triggered with 250 µg recombinant hCG (Ovitrelle; Merck-Serono, Istanbul, Turkey) when a leading follicle of ≥ 19 mm and two or more trailing follicles of ≥ 17 mm were recorded. Follicular aspiration was performed 36 h after ovulation trigger. Oocyte retrieval was performed under general anaesthesia using a double lumen needle (Cook Ireland Ltd., Limerick, Ireland) as we described previously.
Hormone assays
Serum samples for hormone assays were obtained by veni-puncture and assessed using a validated electrochemiluminescence immunoassay (ECLIA method, Cobas 6000, Roche, Basel, Switzerland) as described previously11. Analytical sensitivity (lower detection limit) for P4 was 0.095 nmol/L (0.030 ng/mL) and the functional sensitivity (defined as lowest analyte concentration that can be reproducibly measured with a between-run coefficient of variation [CV] of < 20%) was 0.48 nmol/L (0.15 ng/mL). The day-to-day CV was 2.9% at 2.31 nmol/L (0.73 ng/mL), 1.4% at 9.57 nmol/L (3.1 ng/mL), and 0.9% at 103 nmol/L (32.4 ng/mL). Analytical sensitivity for E2 was 18.4 pmol/L (5 pg/mL). The day-to-day CV for E2 was 6.7% at 27.4 pg/mL, 1.1% at 1270 pg/mL, and 1.9% at 2720 pg/ml. The same assay was used during the study period and was calibrated whenever a new reactive batch was used or whenever an outcome outside the normal range was observed.
Statistical analysis
Continuous variables in the baseline demographic and IVF characteristics were expressed as mean (SD) or median (25th–75th percentile) depending on distribution characteristics. Two-tailed Pearson correlation test and linear regression analysis were used to identify the confounding variables that show significant association with serum P4 level. Continuous variables were compared between the groups with paired samples t- test or Wilcoxon signed rank test as appropriate. The significance level was set at 5% (P < 0.05). Graphpad Prism (version 7) and SPSS statistical programs (version 23) were used to analyse the data and create the figures11.
Results
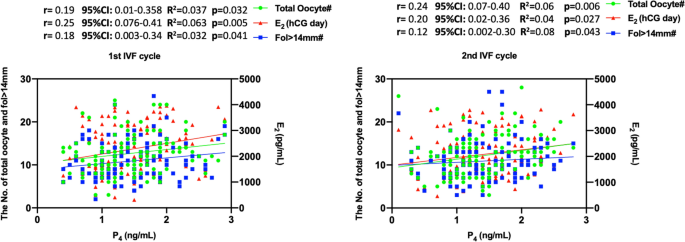
Baseline demographic and IVF characteristics of the two consecutive cycles are summarized in Table 1. Male factor infertility (78.5%) was the major indication followed by unexplained infertility (9.4%), tubal factor (11.9), and ovulatory pathologies (1.6%). Average age, daily and total doses of gonadotropins and duration of stimulation, serum levels of P4 and E2 levels on the hCG day, the numbers of fol > 14 mm on day 10 of stimulation and total and mature oocytes retrieved were similar between the two cycles (Table 1). In both cycles, serum P4 level on the hCG day was significantly associated with serum E2 level on the hCG day, and the numbers of growing follicles (fol > 14 mm) on day 10 of stimulation, and the oocytes retrieved on the correlation and linear regression analyses (Fig. 1). However, the level of significance was not the same for all three co-variates of P4. E2 on the hCG day and total oocyte counts appeared to be more closely associated with P4 in both 1st and 2nd IVF cycles than the number of fol > 14 mm.
The association of P4 level on the hCG day with E2 level on the hCG day, and the numbers of fol > 14 mm and retrieved oocytes on the correlation and linear regression analyses in the 1st and 2nd IVF cycles.
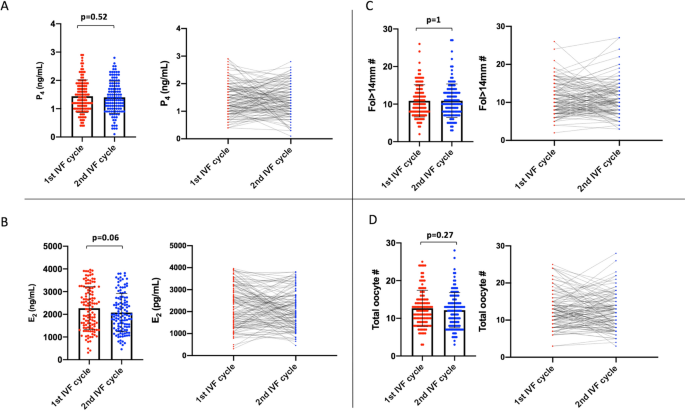
The mean values of P4 and its co-variates (serum E2 level on the hCG day, and the numbers of growing follicles (fol > 14 mm) on day 10 of stimulation, and the oocytes retrieved) were comparable between the cycles. However, we noticed there were substantial cycle to cycle variations in these markers (Fig. 2).
Comparison of the means of P4 on the hCG day (A) and its co-variates E2 on the hCG day (B), and the numbers of fol > 14 mm on day 10 of stimulation (C) and retrieved oocytes (D) between the 1st and 2nd IVF cycles are shown as scatter plot with bars (the left images graphics). The variations in these parameters between the cycles are also shown as lines
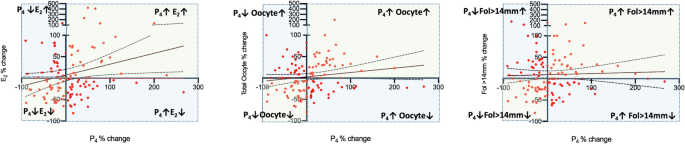
Next, we investigated how serum P4 level and its correlates changed in the 2nd IVF cycle in comparison to their corresponding 1st cycle values in each patient. This was expressed as the percentage of change based on the formula as follows: [(P4 in the 2nd cyle − P4 in the 1st cyle) × 100/P4 in the 1st cyle)]. We observed that there are indeed significant variations in the 2nd cycle when compared to their corresponding 1st cycle values of P4 (%change: − 90.1 to 266.7%), E2 (− 81.7 to 424%), Fol > 14 mm (− 80 to 180%) and oocyte number (− 80 to 200%). The mean, median and percentiles of the percent changes for each of these variables are shown in the Table 2 and depicted as a scatter plot diagram in the Fig. 3A. Then, the IVF cycles were categorized into three groups according to the percent change of P4 in the second IVF cycle as being > 0; = 0 and < 0 in order to investigate how the co-variates of P4 (E2-hCG day, fol > 14 mm and retrieved oocyte counts) changed in relation to a particular change in P4. By doing so we aimed to identify the cycles in which the co-variates of P4 exhibited paradoxical rather than parallel changes with P4. in the subsequent cycles in comparison to the first cycles.
Scatter plot diagrams of the percent changes of P4 on the hCG day and its correlates E2 on the hCG day, and the numbers of fol > 14 mm on day 10 of stimulation and retrieved oocytes overall (A), and when P4 in the second cycle is > 0 (B), < 0 (C) or = 0 (D). Paradoxical changes in the co-variates of P4 (Y-axis) when P4 increased and decreased are visible on the X-axis.
According to the categorization described above serum P4 level on the hCG day was higher in the 2nd IVF cycle than their corresponding 1st cycle levels in 61 of 122 patients (50.0%); remained unchanged in the seven (5.7%) and decreased in the 54 patients (44.3%). The mean increase in P4 in the 2nd cycle was 50.6 ± 53.3% ranging from 4.76 to 266.6%. However, the mean and range of changes in the co-variates of P4 were 20.3% (− 81.8 to 424.6%) for E2; 10.9% (− 61.9 to 150%) for fol > 14 mm; and 11.2% (− 58.3 to 200%) for total oocyte numbers, suggesting that not all elevations in P4 are accompanied by parallel increases in its-covariates in the 2nd IVF cycle (Table 3, Fig. 3B). We found that E2 and the numbers of fol > 14 mm and retrieved oocytes paradoxically decreased in the 55%, 37.7% and 41% respectively, of these 61 patients despite the elevations in P4 levels in their 2nd IVF cycle. The percent decreases ranged from − 2.03 to − 81.8 for E2; from − 6.67 to − 61.9 for Fol > 14 mm; and from − 7.14 to − 58.3 for the oocyte count. The distribution of the parallel and paradoxical changes in the co-variates of P4 in relation to the changes in P4 are depicted as a histogram in the Fig. 4
Histogram depiction of the percent change of P4 in the 2nd cycle (x-axis) and the corresponding percent change in E2, and the numbers of fol > 14 mm and retrieved oocytes (y-axis). Light green areas show the cycles in which there are parallel changes in P4 and its co-variates (E2 and the numbers of fol > 14 mm and retrieved oocytes). Light blue areas demonstrate the cycles in which there were paradoxical changes in P4 and its correlates. Solid line: linear regression, dotted line 95% confidence intervals.
When a similar analysis was done in the 54 out of 122 patients (44.3%) in whom serum P4 was decreased in the 2nd cycle, the mean decrease in P4 was − 34.1 ± 23.3% ranging from − 5.26 to − 90.1% (Table 4). It appeared that E2 and the numbers of fol > 14 mm and retrieved oocytes paradoxically increased in the 42.3%, 45.3% and 40.7% of these 54 patients respectively. The percent increases ranged from 1.60 to 136.3% for E2; from 6.25 to 180 for fol > 14 mm; and from 8.3 to 100 for the oocyte count (Figs. 3C and 4).
Since the number of patients was too small (n = 7) in the group where serum P4 level remain unchanged between the cycles no statistical analysis was conducted (Fig. 3D).
We also investigated if P4 elevation in the previous cycle can predict its occurrence in the next cycle because it was shown that history of P4 elevation can predict its occurrence again in the next cycle independent of ovarian stimulation. When a cutoff level of 1.5 ng/mL was adopted for serum P4 we found that 57 (46.7%) of 122 patients had serum P4 level ≥ 1.5 ng/mL in the 1st cycle. And 32 (56.1%) of these 57 patients continued to have P4 level exceeding this threshold in the 2nd cycle. Interestingly, despite P4 elevations, 14 (43.8%) of these 32 women had paradoxical decreases in the number of retrieved oocytes in their 2nd cycle with the percent decreases ranging from − 7.14 to − 80.0. Similar decreases (− 2.03 to − 65.2%) were observed in the E2 level in 21 of these 32 patients (67.7%).
Most of the patient in this cohort 94/122 (77%) were normal responders (4–15 oocytes in the 1st cycle). Therefore we conducted a subgroup analysis analysis for this group and obtained similar results: Serum P4 level on the hCG day in the 2nd IVF cycle was higher than their corresponding 1st cycle levels in 48 of 94 patients (51.1%), while it remained unchanged in five (5.3%) and decreased in the 54 patients (43.6%). E2 and the numbers of fol > 14 mm and retrieved oocytes paradoxically decreased in the 51.1%, 35.4% and 33.3% of these 48 patients respectively. The percent decreases ranged from − 2.03 to − 81.8 for E2; from − 6.67 to − 57.1 for Fol > 14 mm; and from − 7.14 to − 58.3 for the oocyte count (Supplementary data).
Discussion
We have shown in this study that serum P4 level on the hCG day and its co-variates (E2 on the hCG day and the numbers of growing follicles > 14 mm on day 10 of stimulation and retrieved oocytes) may exhibit significant cycle to cycle variations even if both cycles are consecutive and comparable for the stimulation protocol, the type and dose of gonadotropin and duration of stimulation. The increase in serum P4 level in the 2nd cycle was not always associated with parallel increases in its co-variates. There were some paradoxical inverse changes in these co-variates that were normally supposed to be in a positive association with P4. Even though P4 was significantly associated with E2 and the numbers of fol > 14 mm and retrieved oocytes in each cycle itself, not all decrease or elevations in P4 in the subsequent cycle were accompanied by parallel changes in its co-variates. These findings suggest that the growth and steroidogenic characteristics of antral cohorts in response to exogenous FSH may vary and may not reliably be predictive of the next cycle even if both cycles are comparable and successive.
It is unclear why there are differences between two cohort of antral follicles of two different cycles exposed to FSH at the same dose and duration. Previous studies documented that there might be fluctuations in the number of antral follicles and AMH levels at early follicular phase between the cycles. Inter-cycle variability might not be confined to cohort of growing antral follicles itself as there could be differences in the expression of FSH receptors of granulosa cells of growing follicles and their response to exogenous FSH. Intraovarian actions of FSH and/or the degree of ovarian stimulation might be responsible for premature P output from granulosa cells without luteinization. Steroidogenic activity of the ovarian follicle changes depending on this developmental stage as well as its receptor abundancy and sensitivity. Ovarian stimulation with exogenous FSH is a continuum of multifollicular development with a number of follicles at different stages of development all contributing to the total steroid synthesis at different levels, any given time-point, such as the day of ovulation triggering. Even when the level of steroidogenesis in each granulosa cell or growing follicle does not increase, total steroid synthesis would increase as a factor of increased number of growing follicles and their steroidogenic granulosa cell mass. Another plausible explanation would be the increase in number or sensitivity of FSH and/or LH receptors on the granulosa cells in response to exogenous gonadotropin stimulation. If there are intrinsic differences among the cohorts of antral follicles recruited by FSH in terms of their FSH receptor expression and responsiveness, their growth kinetics and steroidogenic activity may change from cycle to cycle. A particular P4 level at late follicular phase that was reached after achieving a certain magnitude of ovarian response to stimulation by FSH in one IVF cycle might not necessarily produce the same degree of ovarian stimulation and P4 levels in the next cycle. Variations in the level of significance for the co-variates of P4 between the cycles supports this notion while recognizing at the same time that these differences could be related to the relatively small number of subjects in each cycle.
We have recently provided molecular evidence that FSH itself up-regulates the expression and enzymatic activity of the enzyme 3β-hydroxy steroid dehydrogenase (3β-HSD) and promotes P4 output from human granulosa cells and ovarian tissue samples without luteinization9. Therefore, serum P4 level before ovulation trigger is significantly associated with the markers of the degree of ovarian stimulation in multivariate regression analysis, that are the co-variates of P4 and include the number of growing follicles in response to FSH on day 10 of stimulation, E2 level on the hCG day and the numbers of oocytes retrieved.
Data regarding variations in the hormonal profile of women undergoing a similar type ovarian stimulation is limited. A retrospective analysis of 197 women with multiple treatment cycles, showed that premature progesterone elevation is likely to recur in repetitive stimulation cycles (OR 8.4; 95% CI 2.8–24.9). The increased likelihood of recurrence persisted when the regression model was adjusted for the intensity of ovarian stimulation. In the same study, basal P level at the initiation of stimulation was independently associated with P4 levels on triggering day. Authors suggested that in the presence of a corpus luteum that had not undergone functional luteolysis might be responsible from high levels of late follicular phase P4. It was also suggested that persistent high P4 synthesis in repeated cycles might be related with patient-specific intrinsic defects in ovarian or adrenal steroidogenesis18. In our study, 32 of 122 women (26.2%) had persistent high (≥ 1.5) P4 levels in both cycles. Interestingly, 14 (43.8%) of these women had paradoxical decreases in the number of retrieved oocytes in their 2nd cycle with the percent decreases ranging from − 7.14 to − 80.0. Similar paradoxical decreases (− 2.03 to − 65.2%) were observed in the E2 level on the hCG day of the 2nd IVF cycle in 21 of these 32 patients (67.7%). These results provide additional evidence that the co-variates of P4 may not reliably predict its elevation again in the subsequent cycle. A recent prospective study reported significant intra-day variations in serum progesterone levels during the day of ovulation trigger (Gonzales-Foruria et al. 2019). P4 level was highest early in the morning and then gradually decreased throughout the day. Hormone measurements in the blood samples were performed at the same time period of the day early in the morning between 08:00 and 10:00 AM in our study.
These findings were obtained in a relatively small number of IVF patients most of whom were good responders and male factor infertility was the main indication for IVF in majority of the cases. Currently, it is unclear if these results vary depending upon infertility etiology and the types of ovarian stimulation protocol and ovarian response. This is also true when there are accompanying ovarian pathology or other disease that may alter ovarian response to stimulation such as endometriosis, which is a complex disease with genetic, epigenetic and immunologic aberrations. Diminished ovarian reserve or poor response to stimulation are commonly observed in patients with endometriosis undergoing ovulation induction or ovarian stimulation. Despite many efforts we still do not have a serum hormone marker or a correct algorithm to choose the optimal starting dose of FSH in patients with low and high ovarian reserve and in those with PCOS and high AMH. Apparently, bi-directional communication between the oocyte and cumulus granulosa cells plays role in the response to gonadotropins, ovulation, oocyte maturation and IVF success. It is unknown if this bi-directional communication varies from follicle to follicle in a given cycle or between the two consecutive cycles. It also should be remembered that all required steps of controlled ovarian stimulation should be accomplished for best practice in IVF as there are other key factors in addition to premature P4 elevation that might impact the success of IVF such as embryo transfer technique.
To the best of our knowledge this is the first study that analyzed such characteristics of two successive IVF cycles. However, there are several imitations of this study such as its retrospective design, data from a single center, and inclusion of a highly specific group of patients with two consecutive ovarian stimulations cycles within a specified time-period using exactly the same stimulation protocol. Although the design was intended to limit confounding variables like changes in stimulation protocol, dose of gonadotropins and ovarian aging, it limits the number of patients in the study and compromises the generalizability of the findings.
Conclusion
Serum progesterone (P4-hCG day) and its co-variates (estradiol (E2-hCG day) and the numbers of growing follicles > 14 mm and retrieved oocytes) may exhibit significant variations between the two cycles even when both cycles are consecutive and comparable for the stimulation protocol, gonadotropin dose and duration of stimulation. Therefore, the growth characteristics and steroidogenic activities of growing antral cohorts might change from cycle to cycle. The parameters of a previous IVF cycle might not accurately predict the subsequent cycle. These findings need to be confirmed in larger number of IVF patients with different infertility etiologies, ovarian stimulation protocols and ovarian response types.
References
Venetis, C. A., Kolibianakis, E. M., Bosdou, J. K. & Tarlatzis, B. C. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum. Reprod. Update 19, 433–457. https://doi.org/10.1093/humupd/dmt014 (2013).
Martinez, F. et al. Should progesterone on the human chorionic gonadotropin day still be measured?. Fertil. Steril. 105, 86–92. https://doi.org/10.1016/j.fertnstert.2015.09.008 (2016).
Urman, B. et al. Elevated serum progesterone level on the day of human chorionic gonadotropin administration does not adversely affect implantation rates after intracytoplasmic sperm injection and embryo transfer. Fertil. Steril. 72, 975–979 (1999).
Venetis, C. A. et al. Estimating the net effect of progesterone elevation on the day of hCG on live birth rates after IVF: a cohort analysis of 3296 IVF cycles. Hum. Reprod. 30, 684–691. https://doi.org/10.1093/humrep/deu362 (2015).
Griesinger, G. et al. Progesterone elevation does not compromise pregnancy rates in high responders: a pooled analysis of in vitro fertilization patients treated with recombinant follicle-stimulating hormone/gonadotropin-releasing hormone antagonist in six trials. Fertil. Steril. 100(1622–1628), e1621-1623. https://doi.org/10.1016/j.fertnstert.2013.08.045 (2013).
Kyrou, D. et al. The relationship of premature progesterone rise with serum estradiol levels and number of follicles in GnRH antagonist/recombinant FSH-stimulated cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 162, 165–168. https://doi.org/10.1016/j.ejogrb.2012.02.025 (2012).
Ochsenkuhn, R. et al. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: a retrospective study with 2,555 fresh embryo transfers. Fertil. Steril. 98, 347–354. https://doi.org/10.1016/j.fertnstert.2012.04.041 (2012).
Lee, V. C. et al. Effect of preovulatory progesterone elevation and duration of progesterone elevation on the pregnancy rate of frozen-thawed embryo transfer in natural cycles. Fertil. Steril. 101, 1288–1293. https://doi.org/10.1016/j.fertnstert.2014.01.040 (2014).
Oktem, O. et al. FSH Stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Hum. Reprod. https://doi.org/10.1093/humrep/dex010 (2017).
Hill, M. J. et al. Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation?. Fertil. Steril. 103(1477–1484), e1471-1475. https://doi.org/10.1016/j.fertnstert.2015.02.038 (2015).
Oktem, O. et al. High responders are not exempt from detrimental effects of prematurely rising progesterone levels in fresh embryo transfer cycles. Reprod. Biomed. Online 38, 206–215. https://doi.org/10.1016/j.rbmo.2018.11.008 (2019).
Lawrenz, B., Labarta, E., Fatemi, H. & Bosch, E. Premature progesterone elevation: targets and rescue strategies. Fertil. Steril. 109, 577–582. https://doi.org/10.1016/j.fertnstert.2018.02.128 (2018).
Venetis, C. A. et al. Basal serum progesterone and history of elevated progesterone on the day of hCG administration are significant predictors of late follicular progesterone elevation in GnRH antagonist IVF cycles. Hum. Reprod. 31, 1859–1865. https://doi.org/10.1093/humrep/dew141 (2016).
van Disseldorp, J. et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum. Reprod. 25, 221–227. https://doi.org/10.1093/humrep/dep366 (2010).
Depmann, M. et al. Fluctuations in anti-Mullerian hormone levels throughout the menstrual cycle parallel fluctuations in the antral follicle count: a cohort study. Acta. Obstet. Gynecol. Scand. 95, 820–828. https://doi.org/10.1111/aogs.12886 (2016).
Andersen, C. Y. & Ezcurra, D. Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod. Biol. Endocrinol. 12, 128. https://doi.org/10.1186/1477-7827-12-128 (2014).
Bildik, G. et al. Endogenous c-Jun N-terminal kinase (JNK) activity marks the boundary between normal and malignant granulosa cells. Cell Death Dis. 9, 421. https://doi.org/10.1038/s41419-018-0459-3 (2018).
De Geyter, C., De Geyter, M., Huber, P. R., Nieschlag, E. & Holzgreve, W. Progesterone serum levels during the follicular phase of the menstrual cycle originate from the crosstalk between the ovaries and the adrenal cortex. Hum. Reprod. 17, 933–939. https://doi.org/10.1093/humrep/17.4.933 (2002).
Gonzalez-Foruria, I. et al. Clinically significant intra-day variability of serum progesterone levels during the final day of oocyte maturation: a prospective study with repeated measurements. Hum. Reprod. 34, 1551–1558. https://doi.org/10.1093/humrep/dez091 (2019).
Lagana, A. S. et al. The pathogenesis of endometriosis: molecular and cell biology insights. Int. J. Mol. Sci. 20, 5615. https://doi.org/10.3390/ijms20225615 (2019).
Riemma, G. et al. Ion channels in the pathogenesis of endometriosis: a cutting-edge point of view. Int. J. Mol. Sci. 21, 1114. https://doi.org/10.3390/ijms21031114 (2020).
Terzic, M. et al. Ovulation induction in infertile women with endometriotic ovarian cysts: current evidence and potential pitfalls. Minerva Med. 111, 50–61. https://doi.org/10.23736/S0026-4806.19.06346-8 (2020).
Salamun, V., Verdenik, I., Lagana, A. S. & Vrtacnik-Bokal, E. Should we consider integrated approach for endometriosis-associated infertility as gold standard management? Rationale and results from a large cohort analysis. Arch. Gynecol. Obstet. 297, 613–621. https://doi.org/10.1007/s00404-017-4633-0 (2018).
Di Paola, R. et al. Are we choosing the correct FSH starting dose during controlled ovarian stimulation for intrauterine insemination cycles? Potential application of a nomogram based on woman’s age and markers of ovarian reserve. Arch. Gynecol. Obstet. 298, 1029–1035. https://doi.org/10.1007/s00404-018-4906-2 (2018).
Della Corte, L. et al. Current and experimental drug therapy for the treatment of polycystic ovarian syndrome. Expert Opin. Investig. Drugs 1, 12. https://doi.org/10.1080/13543784.2020.1781815 (2020).
Burnik Papler, T. et al. PGR and PTX3 gene expression in cumulus cells from obese and normal weighting women after administration of long-acting recombinant follicle-stimulating hormone for controlled ovarian stimulation. Arch. Gynecol. Obstet. 299, 863–871. https://doi.org/10.1007/s00404-018-5031-y (2019).
Wigglesworth, K. et al. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc. Natl. Acad. Sci. USA 110, E3723-3729. https://doi.org/10.1073/pnas.1314829110 (2013).
Jungheim, E. S., Meyer, M. F. & Broughton, D. E. Best practices for controlled ovarian stimulation in in vitro fertilization. Semin. Reprod. Med. 33, 77–82. https://doi.org/10.1055/s-0035-1546424 (2015).
Cozzolino, M. et al. Ultrasound-guided embryo transfer: summary of the evidence and new perspectives. A systematic review and meta-analysis. Reprod. Biomed. Online 36, 524–542. https://doi.org/10.1016/j.rbmo.2018.01.015 (2018).




.jpeg)



When it comes to the IVF treatment center in Punjab you should choose qualified doctors & affordability, and you can find both of these qualities at Dr Sumita Sofat Hospital.
ReplyDelete